What’s the galvanic couple?
posted in Technical Articles by TecnoConverting
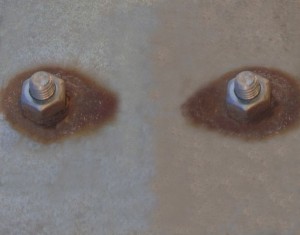
In several installations circular stainless steel scrapers are usually requesting to incorporate joints between two different metals touching each other, such as stainless steel scraper parts and parts in carbon steel. And the galvanic couple causes the appearance of rust.
We consider it´s interesting to inform some important aspects about the galvanic couple:
Wikipedia’s definition: “A galvanic couple is a corrosive cell that is developed when two different metals are separated by electrolytes. In essence, it is the electrochemical action that is produced by the reaction of two dissimilar metals, given there is a path conducive to electrons and electrolytes. It can be identified by the existence of corrosion at a point located between the two metals”.
Important facts to consider:
– Corrosion always appears on the less noble metal, for example, if the galvanized steel is in contact with stainless steel, rust is always displayed in carbon steel.
– Although the contact zone is well isolated between an element and another, it is likely that corrosion also appears through the electrolyte, and moreover if the installation is on the outside, where the environment itself acts as an electrolyte, either by the condensed water in the environment, the dew …
– Another interesting point is the surface of a metal over the other. According to several studies, if the less noble metal is much larger than the noble metal , it seems that the galvanic couple doesn’t act so severely; but also depends on other factors especially atmospherics’ ones and for the presence of other elements in the environment, such as salts and chlorides .
